Do you struggle to maintain a healthy body weight despite diet and exercise? Many have the same challenge and often ask:
How does thermogenesis work to support weight loss?
Thermogenesis affects how your body burns calories and regulates energy balance. It plays a key role in weight management by increasing the metabolism and forcing the body to use fat cells for energy.
At BELDT Labs, we have extensively studied the natural process of fat loss to create high-quality thermogenic supplements like our best-seller, SKALD.
In this article, we'll explain the science of thermogenesis in simple words. You'll understand how it works, the different types of thermogenesis, and how to trigger it to lose weight or even get a shredded body.
Let's start by explaining the thermogenesis in humans.
What is the Process of Thermogenesis in the Human Body?
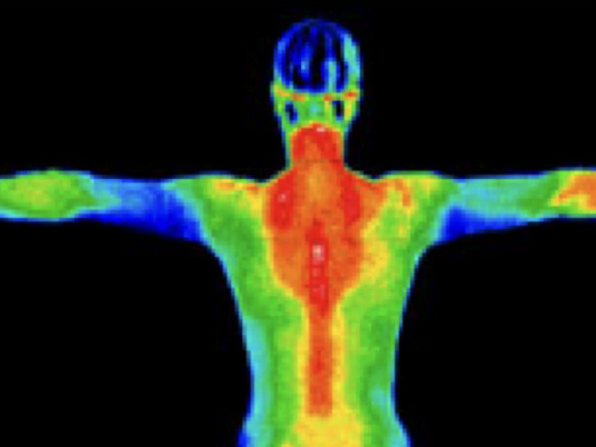
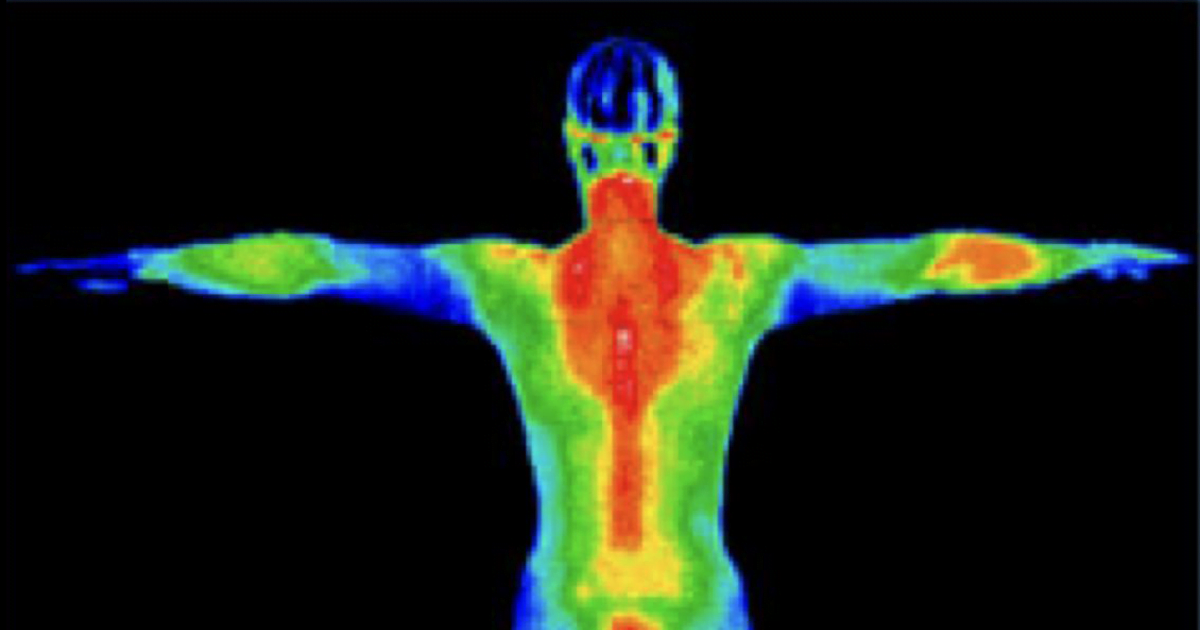
Thermogenesis is the production of heat in the human body. It's a metabolic process that occurs continuously, helping to maintain your core body temperature and regulate your energy balance.
When we talk about thermogenesis in the context of weight loss, we're referring to the body's ability to burn calories to produce heat. This process is ongoing, but certain factors can increase or decrease its intensity.
The primary sites of thermogenesis in our bodies are:
Skeletal muscle, brown adipose tissue (brown fat), liver, and other organs.
These tissues use various mechanisms to generate heat, often involving the breakdown of fatty acids and the activation of specific proteins.
How Thermogenesis Helps You Lose Weight?
Thermogenesis supports weight loss by increasing total daily energy expenditure (TDEE). In simpler terms, it helps you burn more calories throughout the day and reduce body fat. Your TDEE can be easily calculated using science-backed methods.
Here's how it works:
Increased Calorie Burn
When thermogenesis is activated, your body burns more calories to produce heat. This increased energy expenditure can create a calorie deficit, which is essential for fat burning.
Fat Oxidation
Thermogenesis often involves the breakdown of fatty acids for energy. That's a process known as fat oxidation, which is stimulated by oxygen intake and helps you reduce body fat over time. If you want to learn more about the role of oxygen in weight loss, read this article.
Metabolic Rate Boost
Regular activation of thermogenesis can lead to a higher resting metabolic rate, meaning you burn more calories even when at rest.
Utilizing these mechanisms will certainly support your weight loss efforts, but our experience shows that thermogenesis alone isn't a magic solution.
It works better when combined with a balanced diet and regular exercise, and it can be further stimulated with safe weight loss supplements.
What Are the Four Types of Thermogenesis?
We promised to provide comprehensive information on the topic. So, let's break down the four main types of thermogenesis.
Basal Metabolic Rate (BMR)
This is the energy your body uses to maintain basic life functions when you're at complete rest. It accounts for about 60-75% of your total daily energy expenditure.
Exercise-Induced Thermogenesis
As the name suggests, this type occurs during physical activity. Depending on the intensity and duration of the exercise, it can significantly increase your energy expenditure.
Non-Exercise Activity Thermogenesis (NEAT)
These are all activities other than sleeping, eating, or working out. Standing, walking, and everything in between, NEAT accounts for up to 50% of your energy expenditure.
Diet-Induced Thermogenesis
Also known as the thermic effect of food, this is the energy used to digest, absorb, and metabolize your meals. Based on our research, it typically accounts for about 10% of your TDEE.

How to Increase Your Thermogenesis?
We hope you now better understand what thermogenesis is and how it works. So, let's talk about some practical ways to speed up this heat-producing process and burn fat.
Exercise Regularly
Physical activity, especially high-intensity interval training (HIIT) and strength training, can significantly increase exercise-induced thermogenesis. You'll burn many calories during the workout and keep your metabolic rate elevated for hours afterward. We recommend morning workouts for the best results.
Increase Protein Intake
High-protein diets have been shown to increase diet-induced thermogenesis. Protein requires more energy to digest than fats and carbohydrates, leading to a higher thermic effect. Eggs are great for that purpose and won't hurt your budget.
Don't Just Stay Hydrated
Drinking cold water can temporarily boost metabolism, as you use energy to warm the water to body temperature. According to Food Network, a cup of ice-cold water burns an extra 8 calories compared to warm water.
Consume Thermogenic Foods
Certain foods and compounds have been shown to have a thermogenic effect. For the sake of this article, we'll just give you a couple of examples, as these are also effective for appetite suppression:
» Green tea and coffee contain caffeine and catechins, which impact fat metabolism.
» Chili peppers contain capsaicin, which makes you feel full, reducing food intake.
» Coconut oil contains MCTs, which the liver transforms into energy and ketones.
Consider Thermogenic Supplements
For example, high-quality caffeinated green tea supplements can increase your metabolism and energy expenditure to help you lose weight faster.
We go in-depth on safe thermogenic supplements, in another article.
Increase Non-Exercise Activity
Look for ways to move more throughout your day. Take the stairs instead of the elevator, walk while you're on the phone, or use a standing desk. One of the fat loss secrets is as simple as creating habits you can stick to.
Expose Yourself to Cold
Brief exposure to cold temperatures can activate brown adipose tissue, increasing thermogenesis. This could be as simple as taking a cold shower or doing fun outdoor activities in cold weather.
Get Enough Sleep
Lack of sleep can disrupt our hormonal balance and reduce our body's ability to regulate energy expenditure. Some say 7-8 hours is enough, but it depends. If you go to bed late, we recommend aiming for 9 hours.

Thermogenesis and Weight Loss Summary
We hope you found the answers you were looking for. So, let's summarize it.
You now understand that improving your body composition isn't only about cutting calories…boosting your body's natural heat production also helps.
Eat thermogenic foods, stay active, sleep well, and you'll unlock the power of thermogenesis. At BELDT Labs, we encourage you to experiment with these methods and find what suits your body.
If that doesn't bring results…learn about the benefits of our all-natural ingredient thermogenic fat burner.